Welcome to the official website of Hubei ZhengJiu New Materials Technology Co., Ltd.!
γ-Mercaptopropyltrimethoxysilane (KH-580): A Deep Dive into This Multifunctional Silane Coupling Agent
2025-07-18
γ-Mercaptopropyltrimethoxysilane (KH-580): An In-depth Analysis of a Multifunctional Silane Coupling Agent
In the silane coupling agent family, γ-Mercaptopropyltrimethoxysilane (KH-580) stands out as a versatile agent in rubber, composite materials, and metal processing due to its unique structure and properties. While appearing as a complex chemical name, it acts as a bridge between different materials, solving numerous industrial challenges. Today, we will comprehensively explore this amazing silane coupling agent, covering its structure, properties, applications, and synthesis methods.
I. Molecular Structure: Connecting Organic and Inorganic “Dual-Sided Architecture”
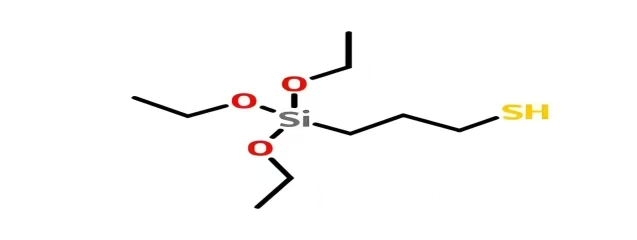
Chemical Name: γ-Mercaptopropyltrimethoxysilane
English Name: γ-Mercaptopropyltriethoxysilane
Molecular Formula: C₉H₂₂O₃SSi
Molecular Weight: 238.42 g/mol
CAS Number: 14814-09-6
The chemical structure of KH-580 is HS-(CH₂)₃-Si-(OC₂H₅)₃. From a molecular perspective, it resembles a "double-sided body": one end is an organic group containing a mercapto group (-SH), and the other end is a siloxane group containing three ethoxy groups (-OC₂H₅).
This structural design is ingenious: the mercapto group has high reactivity and can chemically react with unsaturated bonds in organic materials such as rubber and resin, forming stable chemical bonds; while the ethoxy group is easily hydrolyzed to generate silanol groups (-Si-OH), which can undergo condensation reactions with hydroxyl groups on the surface of inorganic materials such as glass fiber, metal, and ceramics, forming strong covalent bonds. This "organic-loving on one end and inorganic-loving on the other" characteristic makes KH-580 a "molecular bridge" connecting two different types of materials. II. Physical Properties, Solubility, and Chemical Properties: Characteristics Determine Applications
(I) Physical Properties
KH-580 usually appears as a colorless to pale yellow transparent liquid with a characteristic faint mercaptan odor. Its relative density is approximately 0.980-0.995 (25℃), boiling point is between 210-215℃, refractive index is 1.4340-1.4380 (25℃), and flash point (closed cup) is approximately 93℃. These physical parameters make it easy to store, transport, and measure in industrial production, and also provide a basic guarantee for its application under different process conditions.
(II) Solubility
1. Organic Solvents
Easily Soluble: Miscible with most nonpolar/weakly polar solvents, including toluene, xylene, acetone, ethyl acetate, and chloroform.
Partially Soluble: Solubility in saturated hydrocarbons (such as n-hexane) is approximately 5–8 wt% (25℃).
Polar Solvents: Completely soluble in methanol, ethanol, and isopropanol.
The solubility of KH-580 shows a certain selectivity. It is easily soluble in most organic solvents such as ethanol, acetone, toluene, and xylene, and can be uniformly dispersed in these solvents to form stable solutions, making it convenient to use in fields such as coatings and adhesives that require solvent systems.
2. Aqueous Behavior
Insoluble in water, but the ethoxy group (-OCH2CH3) undergoes hydrolysis
Acidic conditions (pH 4–5): Hydrolysis half-life is approximately 2 hours, silanol condensation is slow, and solution stability is high.
Neutral/Alkaline conditions: Hydrolysis is accelerated (half-life <10 minutes at pH 10), and rapid condensation occurs to form Si-O-Si polymer precipitates.
It is insoluble in water; however, it is worth noting that it undergoes slow hydrolysis in water to generate the corresponding silanol. This characteristic is also closely related to its chemical properties and needs to be considered in practical applications. For example, when used in aqueous systems, special emulsification treatment is usually required.
(III) Chemical Properties
In addition to the reactivity mentioned above,
KH-580 also has some important chemical properties. The mercapto group (-SH) has a certain reducibility and is easily oxidized in air to form disulfide. Therefore, it is necessary to isolate it from air during storage and use, avoiding high temperatures and strong light irradiation to prevent its deterioration. In addition, the hydrolysis reaction of the siloxane group is more violent under acidic or alkaline conditions, and the generated silanol groups will also undergo condensation reactions to form siloxane bonds (-Si-O-Si-), which allows KH-580 to form a cross-linked siloxane film when treating the surface of inorganic materials, further enhancing the bonding strength with the material. At the same time, KH-580 can undergo coordination reactions with certain metal ions (such as mercury and silver) to form stable complexes, and this characteristic also provides possibilities for its application in specific fields. III. Core Performance: "All-Round Performance" Stemming from Structure
“All-Round Performance” The performance of KH-580 is closely related to its molecular structure, mainly reflected in the following aspects:
1. Excellent Coupling Ability
1. 优异的偶联能力 This
is the core performance of KH-580. Through the synergistic effect of the two terminal groups, it can effectively eliminate the interfacial tension between organic and inorganic materials and improve the mechanical properties of composite materials. For example, in the composite system of rubber and white carbon black, after adding KH-580, the tensile strength of the material can be increased by more than 30%, and the tear strength can be increased by about 20%.
2. Good Reactivity This
Mercapto group ( At room temperature, -SH can react with a variety of organic compounds, such as addition to double bonds in rubber and coordination with metal ions; the hydrolysis reaction of ethoxy groups can be carried out in water or a humid environment, and the generated silanol groups can quickly combine with inorganic surfaces. This high reactivity makes KH-580 suitable for various process conditions.
3. Excellent Weather Resistance and Stability This
The siloxane structure gives KH-580 good heat resistance and chemical corrosion resistance, and its decomposition temperature can reach above 200℃. In acidic and alkaline environments, it can maintain structural stability and will not easily degrade, so it can be used for material processing in outdoor or harsh environments.
4. Good Dispersibility This
KH-580 can improve the dispersibility of inorganic fillers in organic matrices. For example, adding a small amount of KH-580 to coatings can prevent pigment agglomeration, making the coating more uniform and increasing the gloss by 10%-15%.
IV. Application Scenarios: From Industrial Production to High-End Manufacturing “Versatile”
With its unique properties, KH-580 has penetrated multiple industrial fields, becoming a key auxiliary agent for improving product performance:
1. Rubber Industry: “Performance Enhancer” for Tires and Seals
In tire manufacturing, KH-580 is the best “partner” for white carbon black. It can combine with rubber molecular chains through thiol groups and react with the surface of white carbon black through silanol groups, tightly connecting inorganic fillers and organic rubber, significantly improving tire wear resistance (life extended by more than 20%), tear resistance, and aging resistance. In addition, in the bonding of silicone rubber and metal skeletons, KH-580 can increase the bonding strength by 50%, widely used in seals, shock absorbers, and other products.
2. Composite Materials: “Connecting Bridge” for Glass Fiber and Resin
In fiberglass and carbon fiber composite materials, KH-580 is used to treat the surface of glass fibers, effectively solving the problem of weak bonding between glass fibers and resin matrices. After treatment, the bending strength of the composite material is increased by 30%-40%, the impact strength is increased by more than 25%, and the water resistance and wet heat resistance are significantly improved, often used in wind turbine blades, yacht hulls, and other high-end products.
3. Metal Surface Treatment: “Protective Coating” for Corrosion Prevention and Bonding
KH-580 can form an organosilicon film on the metal surface. This film can isolate water and oxygen and react chemically with subsequent coatings and adhesives, greatly improving the corrosion resistance and coating adhesion of the metal. In the surface treatment of automotive parts and hardware, after KH-580 treatment, the salt spray test time can be extended to more than 500 hours, and the coating peeling rate is reduced by 90%.
4. Coatings and Adhesives: “Secret Weapon” for Performance Enhancement
In coatings, KH-580, as a dispersant and adhesion promoter, can improve pigment dispersibility and the bonding force between the coating and the substrate, making the scrub resistance of the coating 2-3 times higher. In adhesives, adding a small amount of KH-580 can increase the bonding strength of epoxy resin and polyurethane resin to glass and metal by 40%, and significantly enhance weather resistance.
V. Synthesis Method: From Raw Materials to Finished Products “Chemical Magic”
The synthesis of KH-580 belongs to the classic reaction in organosilicon chemistry. Currently, the most commonly used industrial method is the “nucleophilic substitution reaction of mercaptopropyl chloride and triethoxysilane”. The specific process is as follows:
1. Raw Material Preparation
The main raw materials are γ-chloropropyltriethoxysilane (Cl-(CH₂)₃-Si-(OC₂H₅)₃) and sodium hydrosulfide (NaHS). The solvent is usually ethanol or isopropanol, and the catalyst is a quaternary ammonium salt (such as tetrabutyl ammonium bromide).
2. Reaction Principle
Under alkaline conditions, the HS⁻ in sodium hydrosulfide acts as a nucleophile, attacking the chlorine atom (-Cl) in γ-chloropropyltriethoxysilane, undergoing a substitution reaction to generate KH-580 and sodium chloride (NaCl). The reaction formula is as follows:
Cl-(CH₂)₃-Si-(OC₂H₅)₃ + NaHS → HS-(CH₂)₃-Si-(OC₂H₅)₃ + NaCl
3. Process Characteristics
The reaction is carried out at 50-80℃ for 4-6 hours. After distillation purification, the purity of the product can reach more than 98%. This method has readily available raw materials and high yield (up to 90%), but the reaction temperature and pH value must be strictly controlled to avoid the oxidation of thiol groups. In recent years, green synthesis processes have gradually emerged, replacing organic solvents with aqueous reactions, reducing environmental pressure and improving production safety.
VI. Conclusion: The Great Energy of a Small Molecule
γ-Mercaptopropyl triethoxysilane (KH-580), although only a small molecule compound, has built a highly efficient “bridge” between organic and inorganic materials due to its unique “double-sided structure” design. From tires to wind turbine blades, from seals to metal corrosion protection, its applications are ubiquitous, silently supporting the development of high-end manufacturing.
With the continuous improvement of performance requirements in the materials industry, the synthesis process of KH-580 will become greener and more efficient, and its application fields will expand to emerging fields such as new energy and biomedicine. This seemingly ordinary silane coupling agent will release even greater energy in the future.



